Abstract

Introduction: jSSc is a rare autoimmune disease that leads to inflammation, fibrosis, and dysfunction of multiple organ systems which can cause skin sclerosis, interstitial lung disease (ILD), myositis, fasciitis, gastrointestinal and vascular disease. Severe SSc is often refractory to immunomodulatory medications including newer targeted agents. ASCT has demonstrated success in adults with SSc, but experience and data in pediatric patients is limited.
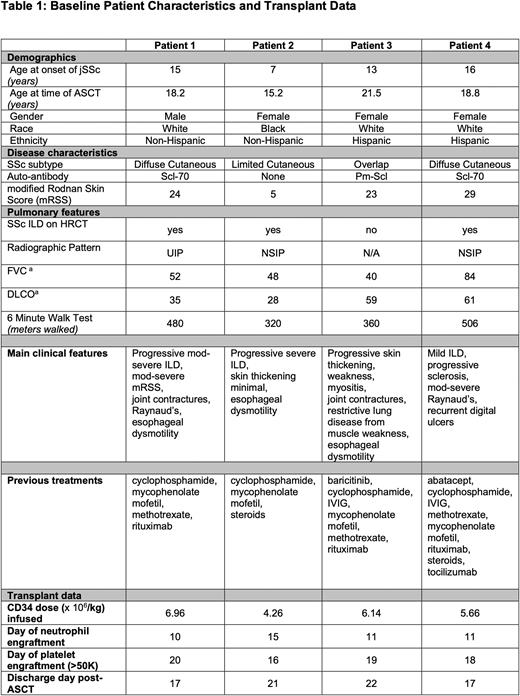
Methods: We report our first four consecutive adolescent and young adult ASCT patients. All patients were referred to our scleroderma center at UPMC Children's Hospital of Pittsburgh where they were evaluated by our multidisciplinary team. Baseline patient characteristics are summarized in Table 1. Patients met criteria for ASCT by severity of lung or skin disease, or both. The first 3 patients had severe ILD or musculoskeletal chest wall weakness resulting in pulmonary function tests (PFTs) below traditional cut offs for adult SSc ASCT trials. All four patients had refractory disease and had previously received 3-8 immunomodulatory medications.
Autologous stem cells were mobilized with cyclophosphamide and filgrastim and collected via pheresis. Following this, conditioning chemoradiation was administered per an IRB-approved individual treatment plan (Patients 1-3) or on our clinical trial NCT03630211 (Patient 4) with rituximab, anti-thymocyte globulin (ATG), TBI 600 cGy with lung and kidney shielding, and cyclophosphamide or thiotepa +/- alemtuzumab. A CD34+ selected cryopreserved autologous peripheral blood stem cell graft was infused on day 0. Antimicrobial prophylaxis was given per institutional standard and PCRs for EBV, CMV and adenovirus were closely monitored. Steroids were weaned off by 5 weeks post-ASCT.
Results: The median cell dose was 5.9 e6 CD34/kg. All patients engrafted, with median neutrophil engraftment on day 11 (range 11-15), and median platelet engraftment (>50K) on day 18.5 (range 16-20). There were infusion-related side effects with equine ATG, but none with rabbit ATG. There were no cases of sinusoidal obstructive syndrome, thrombotic microangiopathy or clinically significant bleeding. No patients required a PCA for pain and received minimal (median=3) doses of narcotics. Patients were discharged from the hospital a median of 19 days after transplant (range 17-21). Only one patient was readmitted, and had a negative 48 hour infectious rule out.
Two patients acquired COVID-19 infections at 5 and 19.5 months post-ASCT; both remained outpatient with quick improvement after treatment with best available therapy. There were no viral reactivations, bacterial or fungal infections.
There has been no evidence of organ toxicity and there are objective improvements in clinical parameters as well as patient reported outcomes (PROs) over the follow-up visits (Table 2). All patients demonstrated an improvement in their skin involvement, as measured by the modified Rodnan Skin Score (mRSS). There was an improvement in clinical symptoms and exam findings of Raynaud's. The lower esophageal sphincter became less hypotensive; objective measures and symptoms of gastroesophageal reflux were much improved. Despite severely reduced values of forced vital capacity (FVC) and diffusing capacity of the lungs for carbon monoxide (DLCO) prior to ASCT, all subjects regained pre-ASCT values. All patients demonstrated an improvement in their PROs and report improvement in fatigue, pain, and the ability to participate in activities of daily living. All patients are off immunomodulatory medications. Tandem serial skin biopsies of the forearm support decreased collagen burden and disappearance of a myofibroblasts population with single cell RNA sequencing (n=3, timepoints 0, 6, 12 mos; data not shown), which correlates with an improvement in the skin score.
Conclusions: In refractory jSSc patients, ASCT is well tolerated, even in patients with severe disease who would otherwise not be eligible for clinical trials based on exclusion criteria that were developed for adults with SSc. ASCT can serve as a hard reset to the immune system for patients with this refractory autoimmune disease, with improvements in clinical parameters that reflect disease modification involving the skin, musculoskeletal, vascular, and gastrointestinal systems. Further clinical research treating patients with this rare disease is of great need.
Disclosures
Szabolcs:Forge Biologics, Inc: Patents & Royalties.
OffLabel Disclosure:
alemtuzumab as part of hematopoietic stem cell transplant conditioning regimen
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal